Antibody For Covid 19 Treatment
Trump receives experimental antibody treatment for Covid-19 diagnosis The treatment from Regeneron is being studied in clinical trials. Thats why mAb treatment may help patients who are at high risk for severe symptoms or having to be hospitalized.

What Are Monoclonal Antibodies And Can They Treat Covid 19 Iav
Both products are combinations of two monoclonal antibodies that each target different parts of the SARS-CoV-2 virus spike protein.
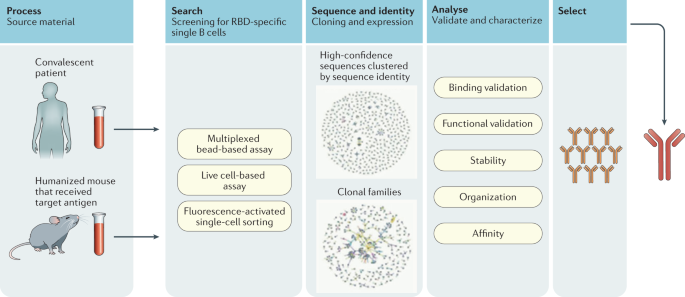
Antibody for covid 19 treatment. Treatment with COVID-19 monoclonal antibodies involves a one-time intravenous IV infusion of a monoclonal antibody product. 1 In addition the FDA recently updated the EUA criteria for all authorized anti-SARS-CoV-2 monoclonal antibodies. MAb treatment can lower the amount of virus in your body reduce symptoms and help avoid a trip to the hospital.
The drug may be used to treat adults and children ages 12 and older and weighing at least 88 pounds who have been hospitalized for COVID-19. Monoclonal antibodies are immune molecules that are produced in a laboratory and designed to mimic the bodys natural response to infection. However depending on your insurance coverage you may need to pay for the administration of.
The antibody therapy AZD7442 reduced the risk of developing symptomatic Covid-19 by only 33 among all exposed patientsincluding those. Vir and GSKs antibody called VIR-7831 was first isolated in. MAb treatment is most effective when received soon after COVID-19 symptoms begin so it is important to get tested right away.
TORONTO -- A Canadian-made COVID-19 antibody treatment is sitting on. SEOUL June 14 Reuters - South Korean drugmaker Celltrion Inc 068270KS on Monday announced positive results for its experimental antibody COVID-19. Monoclonal antibodies or mAbs are made in a laboratory to fight a particular infectionin this case SARS-CoV-2and are given to patients directly with an infusion.
European regulators have recommended antibody treatments by GSK Celltrion Eli Lilly and Regeneron for used in early-stage patients who are at risk of progressing to severe COVID-19. There are currently two products available by FDA Emergency Use Authorization. Monoclonal antibody mAb treatment is for people who have tested positive for COVID-19 and are not sick enough to be in the hospital.
Monoclonal antibody drugs for COVID-19 would normally cost 2000 to 2500 for a single-dose treatment but are currently provided for free by the government. Clinical trials suggest that in these patients remdesivir may modestly speed up recovery time. The NIH however recommend these antibodies for hospitalized COVID-19 patients on the condition that healthcare professionals use them in conjunction with other treatments.
The data supporting this emergency authorization add to emerging evidence that points to the clinical utility of neutralizing antibodies for the treatment of COVID-19 in certain patients. MAb treatment for COVID-19 is different from a COVID-19. In October 2020 the FDA approved the antiviral drug remdesivir to treat COVID-19.
During the phase 2 trials on LY-CoV555 for example a single infusion of 2800 mg of this antibody was found to effectively clear the virus by day 11 following diagnosis with mild to moderate. On May 26 2021 the Food and Drug Administration FDA issued an Emergency Use Authorization EUA for the anti-SARS-CoV-2 monoclonal antibody sotrovimab previously VIR-7831 for the treatment of nonhospitalized patients with mild to moderate COVID-19 who are at high risk of progression to severe COVID-19. Researchers in Texas report in the journal Nature that a COVID-19 antibody treatment they engineered has proved very effective at neutralizing more than 20.
A locally developed treatment for COVID-19 has been given the green light from Ottawa but still hasnt been used in BC. A pharmacist in Chandler Ariz prepares an injection. The resulting monoclonal antibodies have since been tested in a variety of settings as treatments for COVID-19.
In the case of COVID-19 the antibodies are made to recognize and bind to a part of the SARS-Co-V2 virus that enables it to infect human cells.
Antibodies Made In The Lab Show Some Promise For Treating Covid 19 Science News
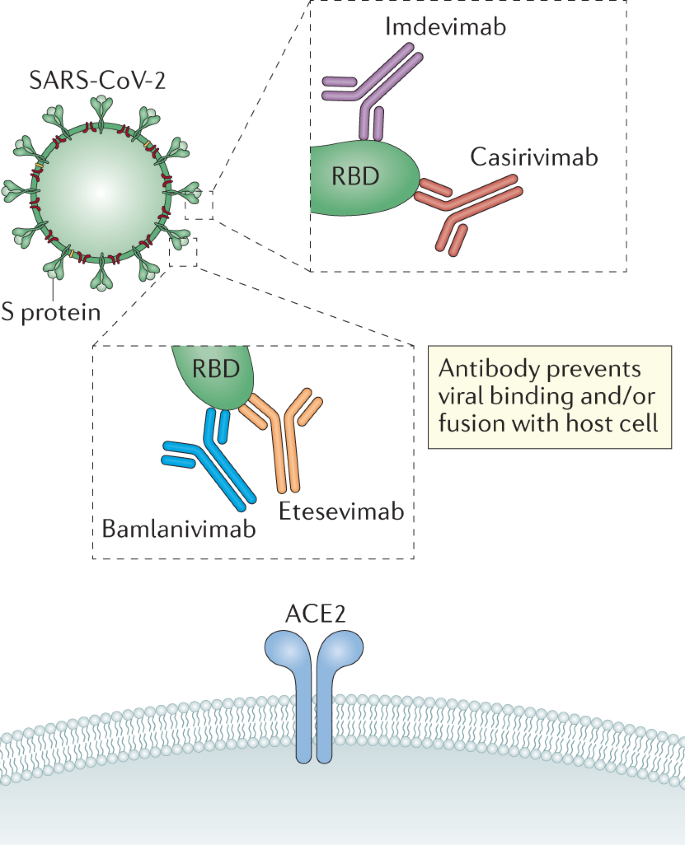
Neutralizing Monoclonal Antibodies For Treatment Of Covid 19 Nature Reviews Immunology
Celltrion Selects 14 Lead Monoclonal Antibodies For Covid 19 Treatment
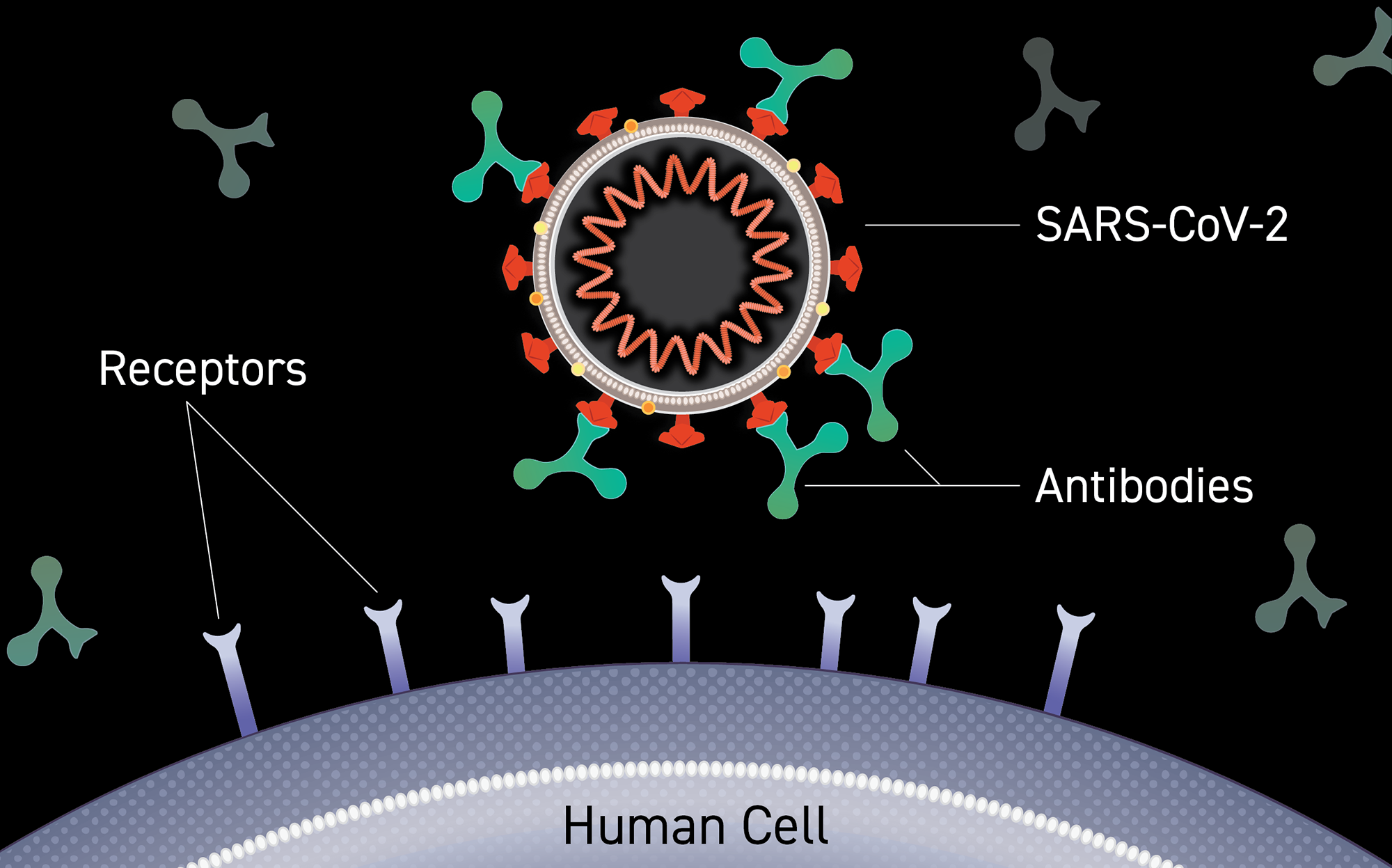
Clinical Trials Of Monoclonal Antibodies To Prevent Covid 19 Now Enrolling National Institutes Of Health Nih

Researching Antibodies To Target Covid 19
How Effective Are Antibodies For Treating Covid 19
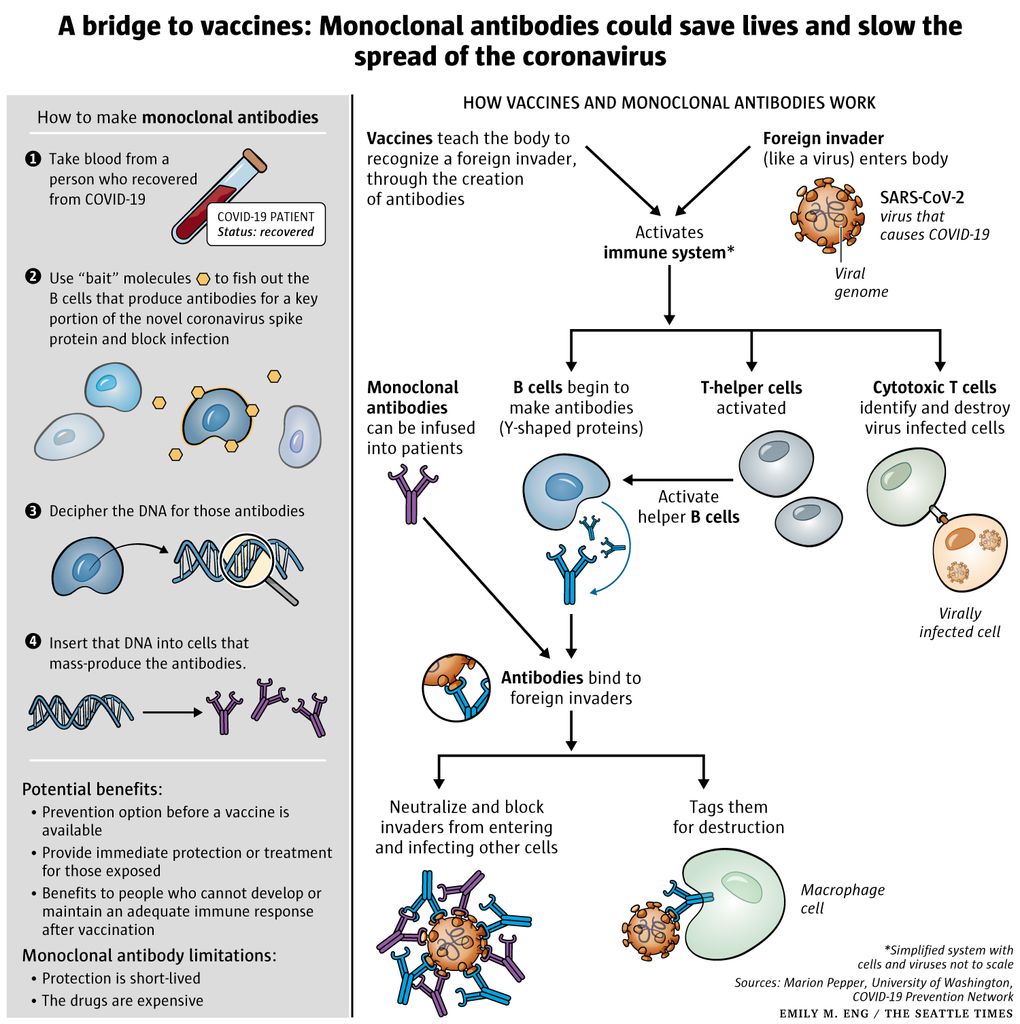
Monoclonal Antibodies Could Fill The Covid 19 Treatment Gap Until Vaccines Arrive But At A Cost The Seattle Times

Neutralizing Monoclonal Antibodies For Treatment Of Covid 19 Nature Reviews Immunology

Provocative Results Boost Hopes Of Antibody Treatment For Covid 19 Science Aaas
Inhaled Antibody Treatment For Covid 19 Shows Pre Clincial Success
Posting Komentar untuk "Antibody For Covid 19 Treatment"