Antibody Treatment For Covid 19 Eli Lilly
Lilly is studying multiple approaches to treat COVID-19 including potential antibodies designed specifically to attack the virus and existing Lilly medicines to understand their potential in treating complications of COVID-19. Clinical trials have shown that etesevimab and bamlanivimab combination treatment and an antibody therapy made by Regeneron seem to significantly reduce hospitalization and deaths among Covid-19.

Eli Lilly Covid Antibody Drug Gets Fda Emergency Clearence Bloomberg
LLY announced today.
Antibody treatment for covid 19 eli lilly. If youve recently been diagnosed with COVID-19 you may have a treatment option. Eli Lilly and Co said on Wednesday that its combination antibody therapy to fight COVID-19 reduced the risk of hospitalization and death by 87 in a. Eli Lilly said its antibody treatment does not work on patients hospitalized with Covid-19.
Europes drug regulator said on Thursday it had initiated a rolling review of US-based Eli Lillys antibodies to treat COVID-19 days after saying their combination could be used in patients at. Stops distributing Lillys COVID-19 antibody therapy in six states instead recommends Regenerons treatment Published. The Food and Drug Administration has authorized Eli Lillys antibody-based drug bamlanivimab for emergency use as a treatment for COVID-19.
About Eli Lilly and Company. Eli Lilly seeks EUA from FDA for Covid-19 antibody treatment. A drug developed by Eli Lilly dramatically reduced the risk of developing symptomatic COVID-19.
The treatment should be given as soon as possible after a positive Covid-19 test result within 10. Eli Lilly says monoclonal antibody cocktail cuts hospitalizations by 70 for high-risk COVID-19 patients. CNNEli Lilly and Companys monoclonal antibody combination therapy helped prevent symptomatic Covid-19 infection among nursing home residents.
AbCellera and Lilly will select from 500 unique antibodies isolated from one of the first US. The trial will look at whether a single dose of the antibody treatment reduces the rate of infection over four weeks as well as symptoms over eight weeks. 9 2020 509 PM PST.
Eli Lilly says its monoclonal antibody cocktail is effective in treating Covid-19. The research so far shows that for certain people this treatment option may help limit the amount of virus in the body. While vaccines may help slow the COVID-19 pandemic over the next months drug company Eli.
The cost per dose. The Food and Drug Administration has granted an emergency use authorization for Eli Lillys Covid. May 28 2021 at 1044 am.
ELI LILLY AND COMPANYMAY 2021 For the Emergency Use Authorization of bamlanivimaband etesevimabtogether for the treatment of COVID-19 The Secretary of the Department of Health and Human Services has declared a public health emergency that justifies the emergency use of bamlanivimaband etesevimabtogether to treat coronavirus disease. Eli Lilly via AP hide caption. Eli Lilly has struck a deal with the federal government to provide 300000 doses of a drug thats designed to keep people infected with COVID-19 out of the hospital.
Eli Lilly receives authorisation for Covid-19 antibody treatment. Monoclonal antibody treatment by Eli Lilly found to cut risk of serious COVID-19 drugmaker reports. Bamlanivimab bam-la-NIV-i-mab and etesevimab e-te-SEV-i-mab administered together.
Eli Lilly will no longer administer its antibody treatment for Covid-19 to hospitalized patients. VANCOUVER British Columbia and INDIANAPOLIS March 12 2020 PRNewswire -- AbCellera and Eli Lilly and Company NYSE. The trial will evaluate the safety and success of using LY-CoV555 a potent neutralizing antibody that works against the spike protein of the novel coronavirus.
Patients who recovered from COVID-19 to create antibody therapeutics for treatment and prevention of COVID-19. The US Food and Drug Administration has given Eli Lilly the first emergency use authorisation for a Covid-19 antibody treatment.

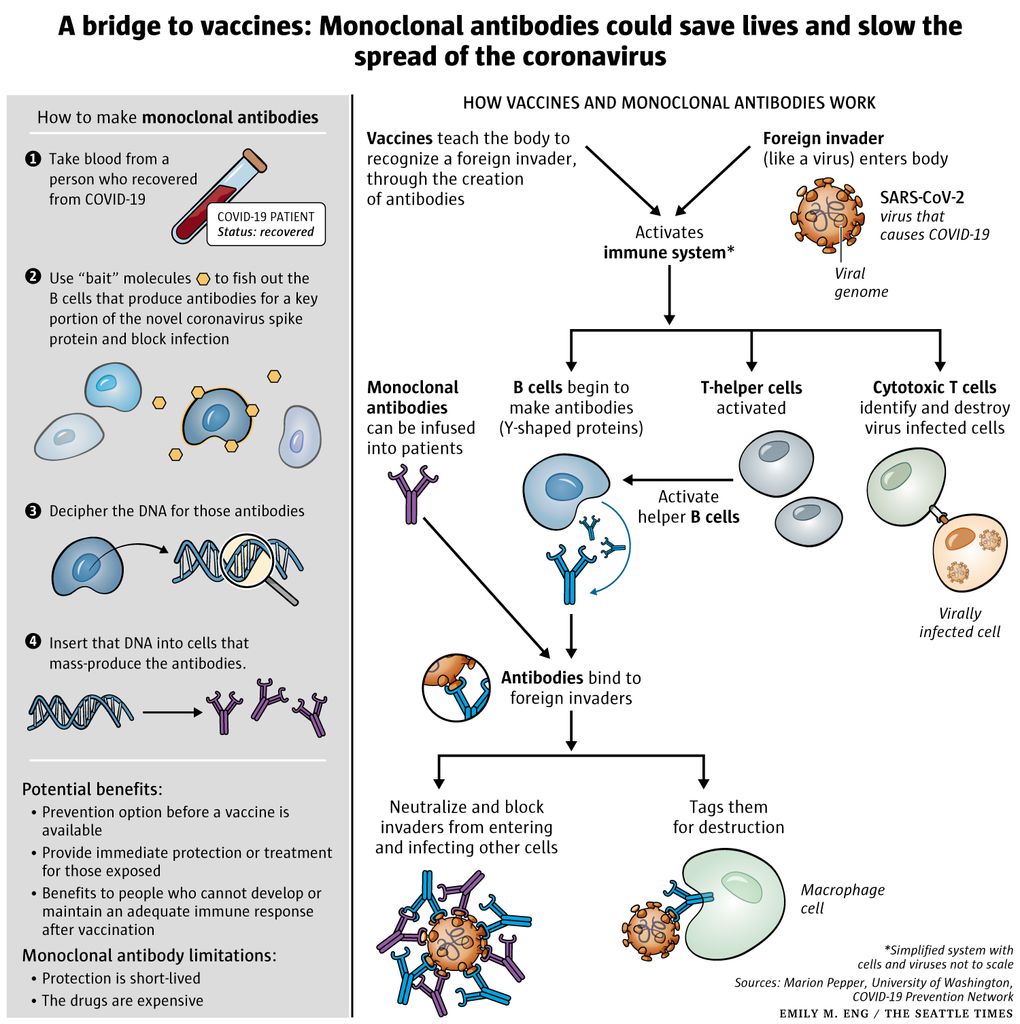
Monoclonal Antibodies Could Fill The Covid 19 Treatment Gap Until Vaccines Arrive But At A Cost The Seattle Times

Fda Gives Emergency Ok To Lilly S Antibody Treatment For Covid 19 Ctv News
Nih Halts Study Exploring Treating Covid 19 With Lilly Antibody Remdesivir Wsj

First Human Trial Of Potential Antibody Treatment For Covid 19 Begins Cnn
Lilly S Covid 19 Antibody Treatment Reduces Death Hospitalizations
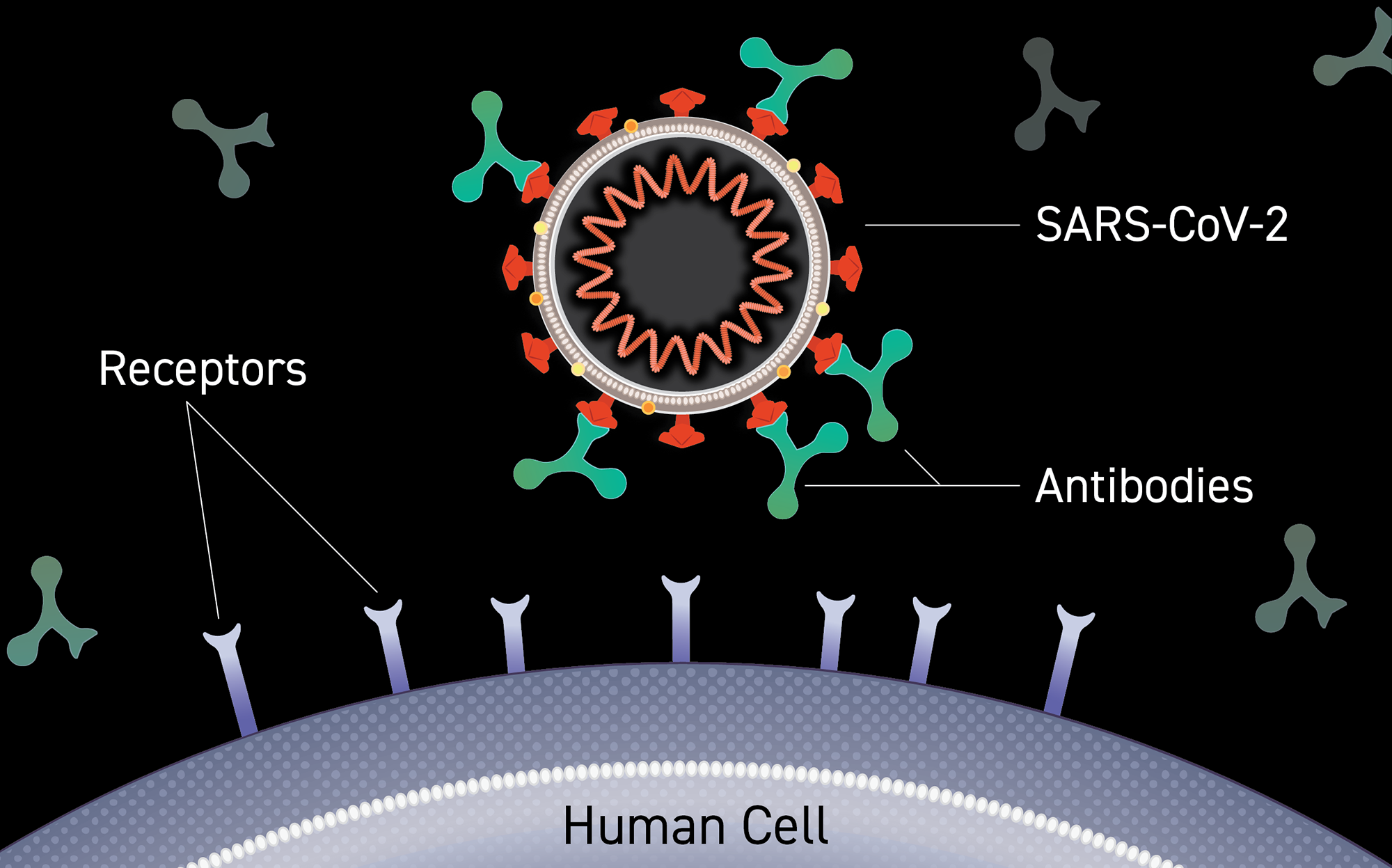
Clinical Trials Of Monoclonal Antibodies To Prevent Covid 19 Now Enrolling National Institutes Of Health Nih
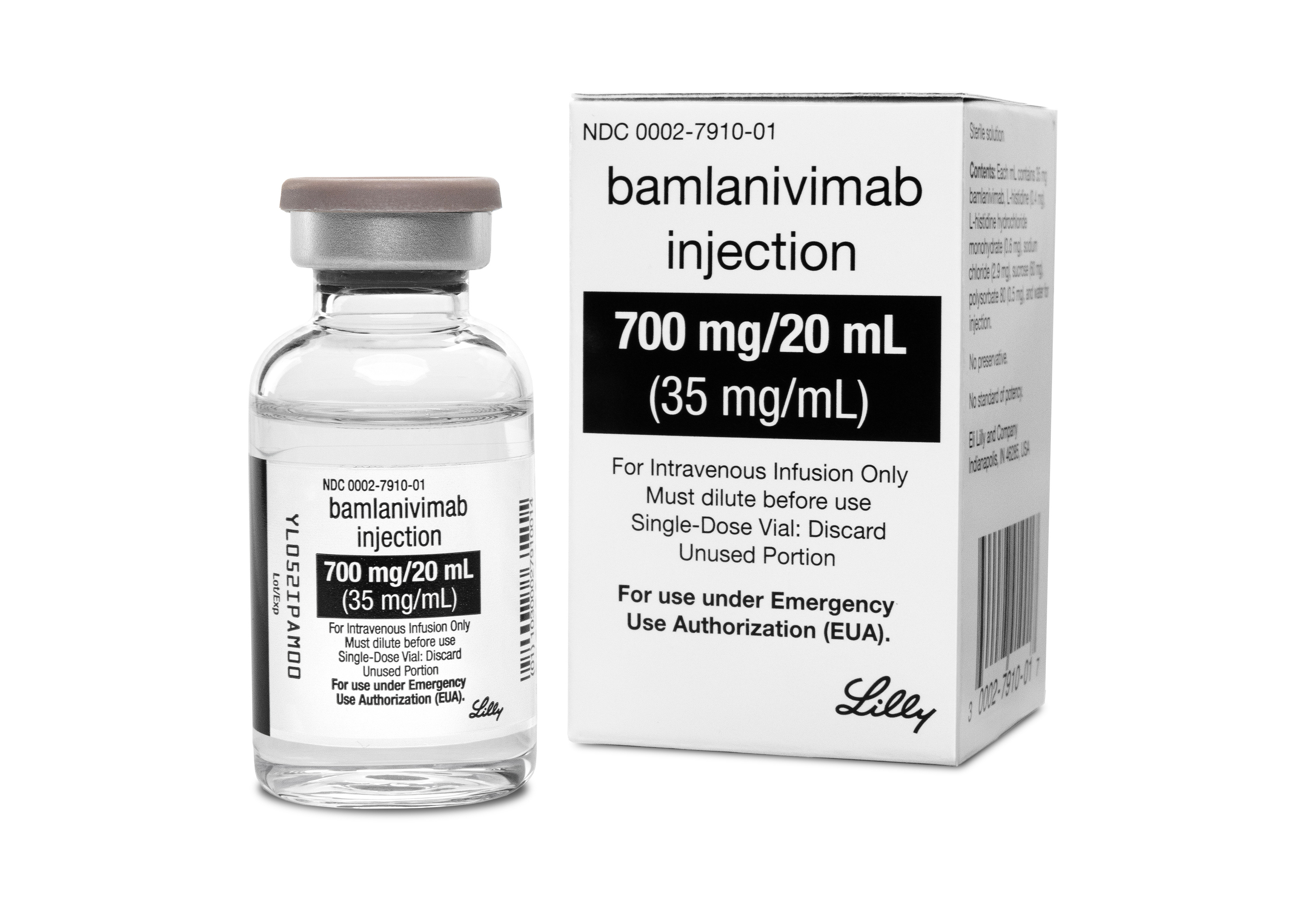
Eli Lilly Antibody Treatment For The Coronavirus Gets Fda S Ok For Emergency Use Shots Health News Npr
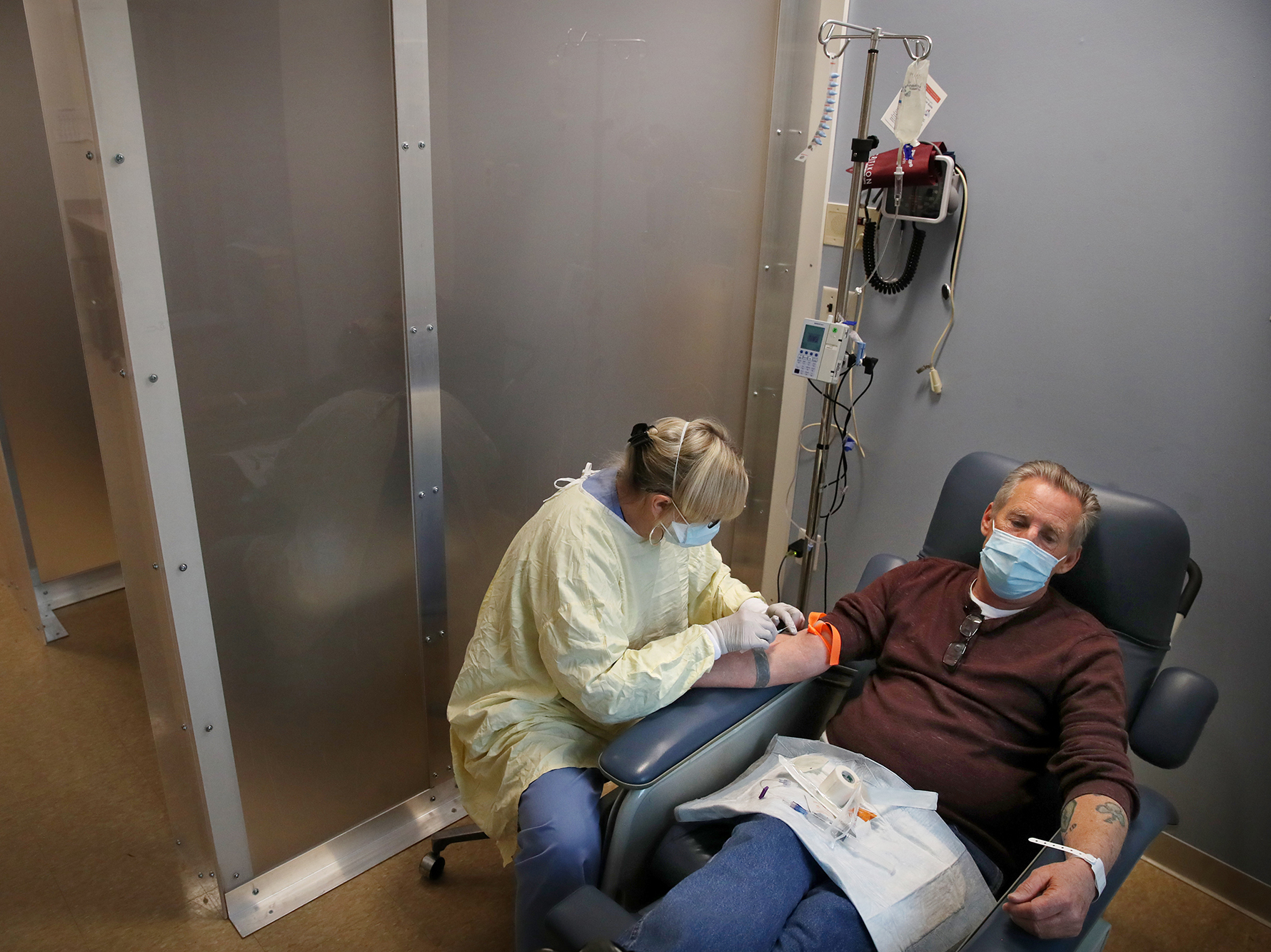
Antibody Treatments For Covid 19 Are Worth The Effort Doctors Say Shots Health News Npr
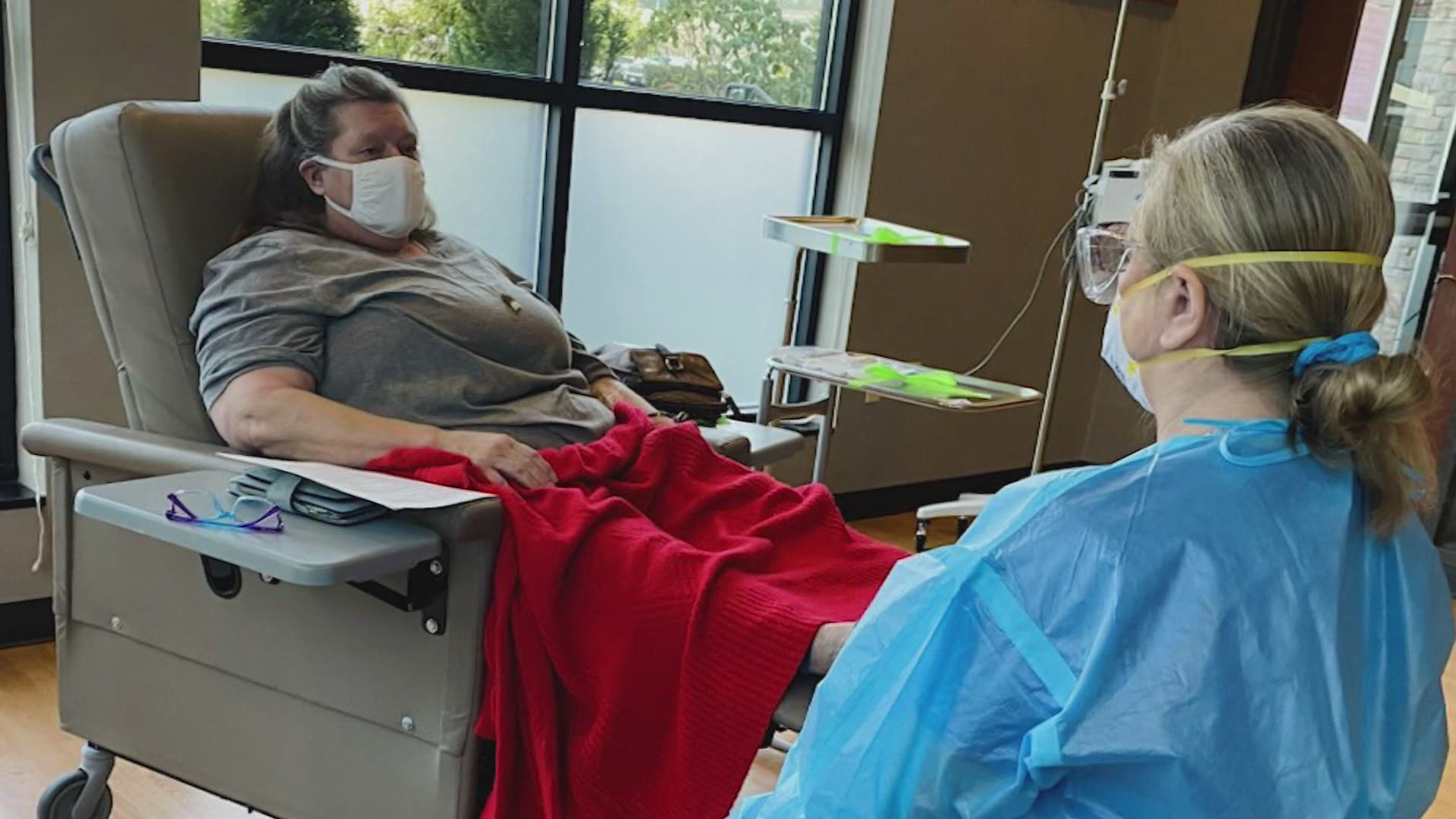
Some Patients In Washington Struggle To Find Covid 19 Antibody Treatment King5 Com

Provocative Results Boost Hopes Of Antibody Treatment For Covid 19 Science Aaas
Posting Komentar untuk "Antibody Treatment For Covid 19 Eli Lilly"